REFERENCES
The Journal of Neuroscience. 2021
Golgi-Dependent Copper Homeostasis Sustains Synaptic Development and Mitochondrial Content
Jan 13;41(2):215-233. doi: 10.1523/JNEUROSCI.1284-20.2020. Epub 2020 Nov 18
Journal of Cellular Physiology 2006
Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic Oct;209(1):183-98. doi: 10.1002/jcp.20722
The Journal of Neuroscience. 2012
Abnormal synaptic vesicle biogenesis in Drosophila synaptogyrin mutants
Dec 12;32(50):18054-67, 18067a. doi: 10.1523/JNEUROSCI.2668-12.2012.
Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains
Oct 19;187(2):295-310. doi: 10.1083/jcb.200903098. Epub 2009 Oct 12.
The Journal of Neuroscience. 2010
DMob4/Phocein regulates synapse formation, axonal transport, and microtubule organization
Apr 14;30(15):5189-203. doi: 10.1523/JNEUROSCI.5823-09.2010.
Molecular and Cellular Neuroscience. 2013
Jan;52:161-72. doi: 10.1016/j.mcn.2012.11.009. Epub 2012 Nov 16.
Highlight
The Journal of Neuroscience. 2021
Golgi-Dependent Copper Homeostasis Sustains Synaptic Development and Mitochondrial Content
Jan 13;41(2):215-233. doi: 10.1523/JNEUROSCI.1284-20.2020. Epub 2020 Nov 18
Cover Article

This Week in The Journal
-
Cellular/Molecular
Complexin Influences Ca2+ Dependence of Vesicle Release
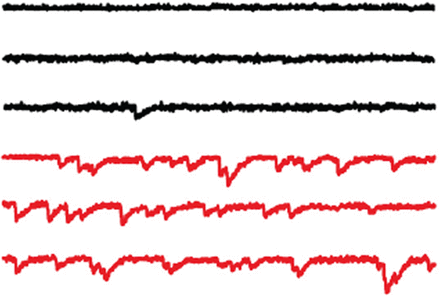
Fusion of vesicles to the plasma membrane is driven by formation of SNARE complexes composed of proteins residing in vesicular and target membranes. Formation of this complex brings the membranes together and delivers energy sufficient to drive fusion. Fusion can occur spontaneously, but at synapses, complexin clamps vesicles when the SNARE complex is partially formed; then, when calcium enters the terminal, synaptotagmin drives synchronous fusion of multiple vesicles. Experiments by Jorquera et al. demonstrate how complexin and synaptotagmin cooperate to regulate spontaneous (spike-independent) and evoked vesicle release in Drosophila larvae. Knocking out complexin increased the frequency of spontaneous vesicle release at neuromuscular junctions, thus reducing the size of the readily releasable pool and the proportion of vesicles released synchronously during spikes. Surprisingly, complexin knockout also reduced the calcium dependence of release. Furthermore, knocking out synaptotagmin rescued spontaneous release rates in complexin-null larvae. Altogether, the results suggest that synaptotagmin can facilitate vesicle fusion independently of calcium, but complexin makes synaptotagmin–SNARE interactions calcium dependent.

The frequency of miniature EPSCs, representing spontaneous vesicle release, is greatly increased at neuromuscular junctions of fly larvae lacking complexin (red) compared with wild-type larvae (black). See the article by Jorquera et al. 2012 for details